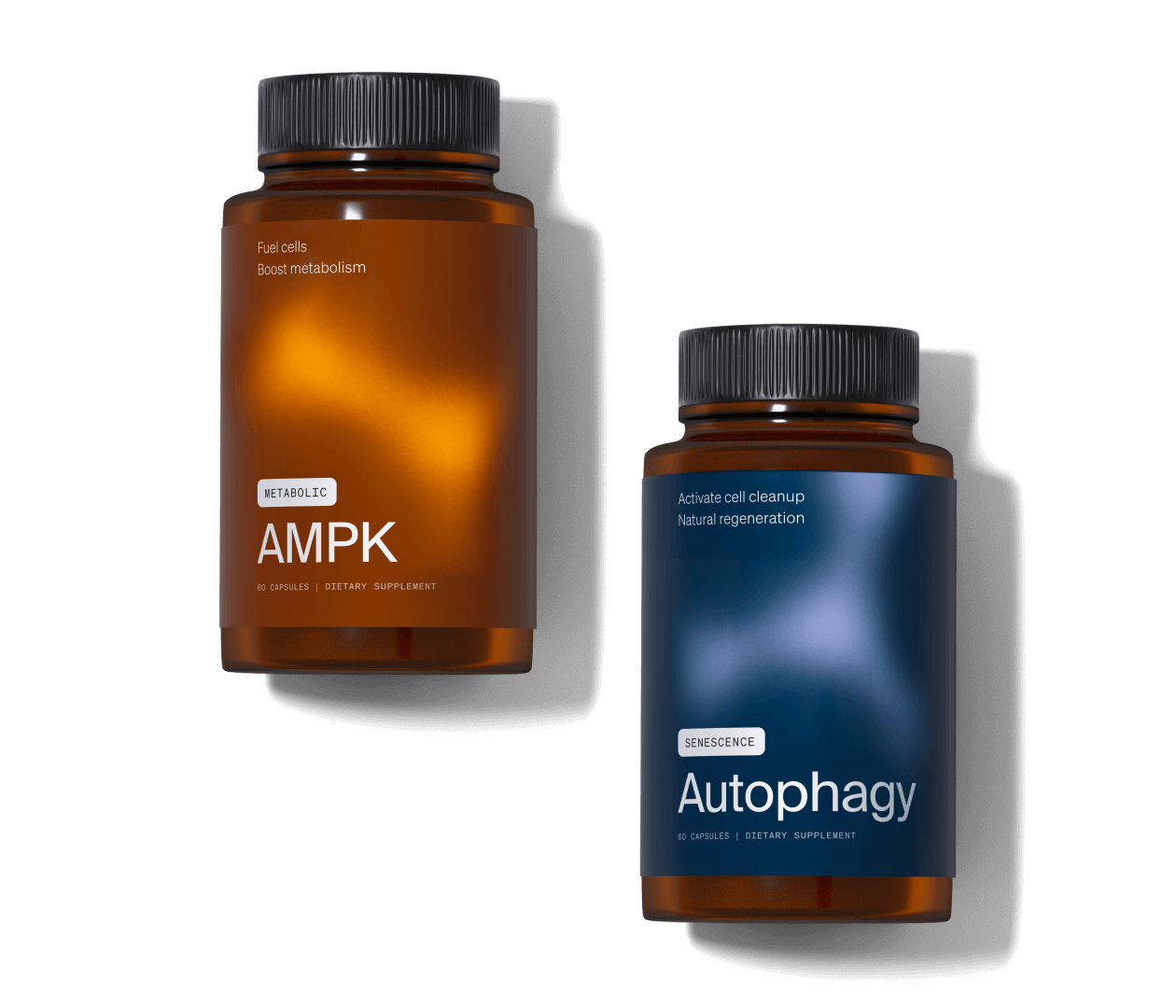
The Science
A main driver of aging is a process called cellular senescence. By understanding how senescent cells form, accumulate, and attack healthy cells, we can affect our rates of aging and mitigate factors for age-related disease.

What is senescence?
(SIH-NESS-UHNS)
Just like us, our cells are busy. Healthy cells divide, replicate, and grow. Dead cells are discarded and used parts recycled. But some cells end up neither healthy nor dead. Scientists call them senescent (or “zombie”) cells.
What it means for healthspan

Interventions can help
Block inflammatory signals
Distressed cells emit signals to convert healthy cells and damage tissue. Interrupting these signals reduces chronic inflammation.


Reduce harmful cells
Senescent cells disrupt healing, so targeting them helps us maintain muscle strength, skin elasticity, and organ function as we age.
Support cell renewal
Interventions that support autophagy, the body’s natural cleansing process, improve repair, renewal, and resilience on a cellular level.

Reduce harmful cells
Senescent cells disrupt healing, so targeting them helps us maintain muscle strength, skin elasticity, and organ function as we age.

Block inflammatory signals
Distressed cells emit signals to convert healthy cells and damage tissue. Interrupting these signals reduces chronic inflammation.

Support cell renewal
Interventions that support autophagy, the body’s natural cleansing process, improve repair, renewal, and resilience on a cellular level.
our understanding
Going deeper into the science behind cellular senescence
Targeted programs
Frequently asked questions
Lowering inflammation through healthy lifestyle habits like exercise, stress reduction, and an anti-inflammatory diet helps reduce the harmful effects of senescent cells. However, these approaches do not remove the cells themselves. Senescent cells are metabolically active and resistant to death, continuing to release damaging signals. Senescence protocols combine lifestyle support with targeted therapies like Rapamycin and senolytics, which help actively clear senescent cells from tissues and restore the body's regenerative capacity.
While autophagy is a natural process, it becomes less effective with age. As damaged proteins and organelles accumulate, they overwhelm the body's ability to maintain cellular integrity. Interventions like Rapamycin help reactivate autophagy by supporting pathways that restore internal cleanup mechanisms, reducing the burden that contributes to aging and dysfunction.
Senolytics work by selectively eliminating senescent cells, removing the source of harmful inflammatory signals. Autophagy enhancers, on the other hand, improve the cell’s ability to break down and recycle damaged internal components. Together, these approaches address both the accumulation and internal debris that drive aging, offering a more complete strategy for cellular renewal.
Yes. While senescent cells do accumulate with age, they can also build up earlier in life due to chronic inflammation, oxidative stress, metabolic dysfunction, or environmental exposures. In these cases, targeted interventions such as Rapamycin or autophagy-supporting protocols can help reduce cellular burden, enhance tissue repair, and preserve long-term health.
Yes. Accumulated senescent cells contribute to systemic inflammation, tissue stiffness, and impaired recovery. These issues can manifest as fatigue, joint discomfort, and skin changes. Interventions that clear senescent cells and restore cellular cleanup often lead to noticeable improvements in energy, mobility, and resilience over time.
Lowering inflammation through healthy lifestyle habits like exercise, stress reduction, and an anti-inflammatory diet helps reduce the harmful effects of senescent cells. However, these approaches do not remove the cells themselves. Senescent cells are metabolically active and resistant to death, continuing to release damaging signals. Senescence protocols combine lifestyle support with targeted therapies like Rapamycin and senolytics, which help actively clear senescent cells from tissues and restore the body's regenerative capacity.
While autophagy is a natural process, it becomes less effective with age. As damaged proteins and organelles accumulate, they overwhelm the body's ability to maintain cellular integrity. Interventions like Rapamycin help reactivate autophagy by supporting pathways that restore internal cleanup mechanisms, reducing the burden that contributes to aging and dysfunction.
Senolytics work by selectively eliminating senescent cells, removing the source of harmful inflammatory signals. Autophagy enhancers, on the other hand, improve the cell’s ability to break down and recycle damaged internal components. Together, these approaches address both the accumulation and internal debris that drive aging, offering a more complete strategy for cellular renewal.
Yes. While senescent cells do accumulate with age, they can also build up earlier in life due to chronic inflammation, oxidative stress, metabolic dysfunction, or environmental exposures. In these cases, targeted interventions such as Rapamycin or autophagy-supporting protocols can help reduce cellular burden, enhance tissue repair, and preserve long-term health.
Yes. Accumulated senescent cells contribute to systemic inflammation, tissue stiffness, and impaired recovery. These issues can manifest as fatigue, joint discomfort, and skin changes. Interventions that clear senescent cells and restore cellular cleanup often lead to noticeable improvements in energy, mobility, and resilience over time.
We study the studies
Healthspan longevity experts continuously review the latest clinical research, curating results to help deepen your understanding of aging science.